Low-speed handpiece infection control: Accountability and care standard
Sean G. Boynes, DMD, MS
Low-speed dental handpieces are universal among dental offices. Dental care teams must implement appropriate care of these regularly used instruments to maintain device integrity and ensure patient safety. It is in the area of patient safety that the profession has experienced higher scrutiny by the media, patients, health-care agencies, and advocacy groups due to reports of infection control failures.1-3 This has led the profession to improve awareness of decontamination and sterilization best practices. The dental profession is becoming evermore risk averse, and patients’ awareness of infection control is as high as it has ever been in the history of dentistry. Thus, it has become a vital business operation to have a team-based understanding and implementation of handpiece sterilization and infection control. This article explores the science, practice regulations, and care standards of infection control management of low-speed dental handpieces.
Analyses of handpiece contamination
In the last 20 years, dental scientists argued that low-speed handpieces can serve as a cross-contamination threat in the dental office due to their design, use, and the type of infection control methodology employed.4-8 Pivotal studies completed at the Indiana University School of Dentistry reported that microbial contamination was demonstrated with the internal components of low-speed handpieces and that internal contaminants can be discharged during use.5-7 Low-speed handpieces feature venting designs to reduce excessive heat buildup and operate as unsealed structures. This necessary design creates an opportunity for microbial emission from the internal areas contaminated during use.5 Therefore, external cleaning or wiping, as a means of decontamination, would not completely thwart conceivable cross-contamination.
Rules, regulations, and liability
New guidelines and changes to state policies and regulations followed the publication and dissemination of these pivotal scientific studies. The Centers for Disease Control (CDC) cited the aforementioned studies when issuing updated guidelines for infection control in dental health-care settings.9,10 The CDC stated that handpieces and their attachments “should always be heat sterilized after each patient.”10 In relation to infection control procedures, they affirm the following: “do not surface-disinfect, submerge in liquid chemical sterilants, or barrier protect these instruments because these methods cannot adequately clean, disinfect, or sterilize the internal components.”10 In addition, the agency goes on to clarify that if this “item is heat-sensitive, dental health-care personnel should replace it with a heat-tolerant or disposable alternative.” The American Dental Association (ADA) has recognized the CDC guidelines and in a position statement said that “sterilization is recommended for all high-speed dental handpieces, low-speed handpiece components used intraorally, and reusable prophylaxis angles.”11
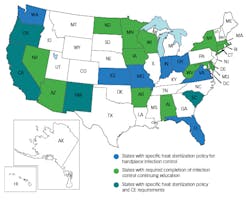
It should also be noted that the Organization for Safety, Asepsis and Prevention (OSAP) reports that 11 states put forth specific policy statements requiring heat sterilization of all handpieces between patients within their state dental practice acts.12 The states requiring low-speed handpiece heat sterilization are highlighted in Figure 1. The remaining 39 states directly or indirectly provide the CDC guidelines as recommendations for infection control in dental practice. Ultimately the structure of dental practice is governed by the individual state dental boards and legislative processes; therefore, each practitioner should review and remain up-to-date with his or her state’s dental rules and regulations. This information is usually available at the state dental board’s website.
Figure 1: States with heat sterilization policy and/or infection control continuing education requirements
Adoption and implementation
While the dental profession has observed universality and consensus with infection control procedures for high-speed handpieces (i.e., heat sterilization after each use), there still remains variation in low-speed sterilization processes.13,14 This lack of agreement may relate to professional judgment, given no documented cases of actual disease transmission have been reported, or the negative impact this process can have on operational business costs.14,15 Another reason for the lack of consensus may relate to the knowledge of and attitudes toward infection control by dental professionals. Gordon et al. reported a systematic review on infection control guidelines and stated that the knowledge of dentists and dental care teams needs to be updated.16 As a means to update knowledge and improve adherence, Garland proposed a workplace environment emphasizing safety and ongoing education.17 In the survey of US dental hygienists, it was revealed that a culture of safety at places of work appeared to be a factor in compliance with infection control guidelines.17 Additionally, McCarthy et al. found that continuing education attendance was a strong predictor of high-level compliance among dentists.18 Twenty states have adopted regulation requiring a specific number of continuing education hours be specific to infection control training, and the number of states with this policy has increased in recent years (figure 1).19
Patient expectations
Infection control and sterilization are regularly deliberated by patients when choosing care and are considered fundamental to their impression of quality health care.1-3 Many dental practices have felt obligated to increase infection control transparency. Sterilization practices and the equipment used have been “anecdotally proposed as the new glove in dentistry.”1,20 A previous analysis found that patients place a higher expectation on overall cleanliness as well as sterilization of instruments compared to other service parameters.21 Specific to handpiece care, a recent CNN report stated that “instruments should be sterilized in between each patient, including the dental drill,” and encouraged patients to ask dental care teams whether this has been completed.2 As I previously stated in Dental Economics, “Undesirable events around infection control can impact patient choice and may result in negative information being posted online and quickly spread via social media.”1 Negative reviews can affect revenue and patient utilization. While one approach to this heightened awareness involves basic activities to decrease liability, some practices are embracing infection control as a marketing opportunity—they are advertising innovations in sterilization and infection control being employed, and that the practice is dedicated to this vital aspect of dental care either through online tours of sterilization areas and equipment, or touting updates in care during patient visits to the office.
A care standard
The CDC recommendations discussed in this article are considered category 1C, which is defined as required by state or federal regulation or representing an established association standard. While professional consensus with low-speed handpiece sterilization processes has not reached complete universal agreement, unless new research contesting the information presented here is accepted and adopted by oversight agencies, the CDC recommendation serves as a disseminated care standard.
References
1. Boynes SG. Exploring the business advantages of efficient instrument processing. Dent Econ. 2017;107(6):55-58.
2. Burhenne M. 5 Things to Do at the Dentist’s Office. CNN website. http://www.cnn.com/2013/03/29/health/dentist-5-things/. Published April 4, 2013. Accessed July 17, 2017.
3. Martinez TS, Hostetler J, Tjahjono J, Bernardo M. Survey Analysis of Parental Awareness and Behaviors Regarding Children’s Dental Care and Dental Infection Control. Western University of Health Sciences. 2015.
4. Waskow JR, Miller CH, Palenik CJ. Justification for sterilizing slow-speed handpiece motors between patients (abstract 3182). J Dent Res. 1996;75(special issue):415.
5. Chin JR, Miller CH, Palenik CJ. Internal contamination of air-driven low-speed handpieces and attached prophy angles. J Am Dent Assoc. 2006;137:1275-1280.
6. Herd S, Chin J, Palenik CJ, Ofner S. The in vivo contamination of air-driven low-speed handpieces with prophylaxis angles. J Am Dent Assoc. 2007;138(10):1360-1365.
7. Chin JR, Westerman AE, Palenik CJ, et al. Contamination of handpieces during pulpotomy therapy on primary teeth. Pediatr Dent. 2009;31(1):71-75.
8. Harte JA, Molinari JA. Sterilization procedures and monitoring. In: Molinari JA, Harte JA, eds. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore: Lippincott Williams & Wilkins; 2009:148-170.
9. Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities. https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf. Published 2008. Updated February 15, 2017. Accessed July 14, 2017.
10. Division of Oral Health, National Center for Chronic Disease Prevention and Health Promotion. Dental handpieces and other devices attached to air and waterlines. CDC website. https://www.cdc.gov/oralhealth/infectioncontrol/questions/dental-handpieces.html. Updated March 26, 2016. Accessed July 14, 2017.
11. American Dental Association. Sterilization and disinfection of dental instruments. ADA website. http://www.ada.org/~/media/ADA/Member%20Center/FIles/cdc_sterilization.ashx. Published 2004. Updated July 2009. Accessed July 16, 2017.
12. Organization for Safety, Asepsis, and Prevention. Frequently asked questions (FAQs) on dental infection control. http://www.osap.org/?FAQ_Instrum_Ster1#doanystatesrequire. Accessed July 14, 2017.
13. Lewis DL, Arens M, Appleton SS, et al. Cross-contamination potential with dental equipment. Lancet. 1992;340(8830):1252-1254.
14. Garland KV. Separate the facts from fiction. Dimen Dent Hyg. 2012;10(12):44-48.
15. Kelsch N. Sterilizing handpieces. RDH. 2011;31(11):97.
16. Gordon BL, Burke FJ, Bagg J, Marlborough HS, McHugh ES. Systematic review of adherence to infection control guidelines in dentistry. J Dent. 2001;29(8):509-516.
17. Garland KV. A survey of U.S. dental hygienists’ knowledge, attitudes, and practices with infection control guidelines. J Dent Hyg. 2013;87(3):140-151.
18. McCarthy GM, Koval JJ, MacDonald JK. Compliance with recommended infection control procedures among Canadian dentists: results of a national survey. Am J Infect Control. 1999;27(5):377-384.
19. States with infection control training requirements. Infection Control and Patient Safety Training Solutions website. http://icprofessor.com/StateWDentalReq.html. Accessed July 16, 2017.
20. Amos J. Sterilization Center Area [VIDEO] – Is It the New “Gloves?” How to Open a Dental Office website. http://howtoopenadentaloffice.com/sterilization-center/. Published October 23, 2014. Accessed July 14, 2017.
21. Chang WJ, Chang YH. Patient satisfaction analysis: Identifying key drivers and enhancing service quality of dental care. J Dent Sci. 2013;8:239-247.
Sean G. Boynes, DMD, MS, is the director of interprofessional practice at the DentaQuest Institute in Westborough, Massachusetts, overseeing national programs and initiatives focused on the integration and coordination of health care. A prolific speaker and author, he has been recognized by many organizations for his work in health policy and clinical care. He can be reached at [email protected].