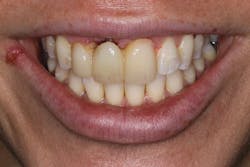
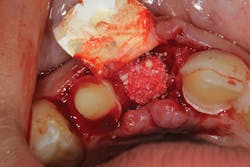
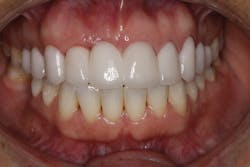
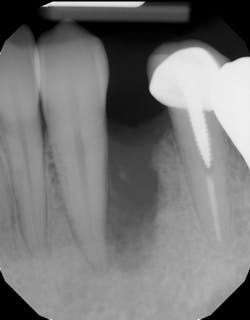
Bone grafting after tooth removal: Why, when, and what to use
Why bone grafting?
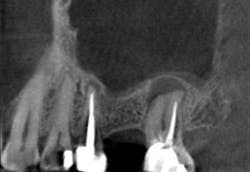
Although unpredictable, a greater amount of alveolar ridge loss following extraction usually occurs in the horizontal dimension and affects the buccal bone of the ridge.3 In fact, 50% of alveolar bone dimension can be lost after tooth extraction, with losses reported of up to 6–7 mm (figure 1). Two-thirds of this loss of bone volume can occur within the first three months of tooth extraction.4
When to use bone grafting?
Because of this alveolar resorptive pattern after tooth extraction, bone grafting the extraction socket after tooth extraction procedures has become a solution that attempts to limit the amount of hard- and soft-tissue loss. There are many systematic reviews in the literature that compare the results of residual ridge dimension following tooth extraction after the use of a bone graft (with or without a membrane) versus extraction alone without grafting.7
Indications for bone grafting extraction sites include:
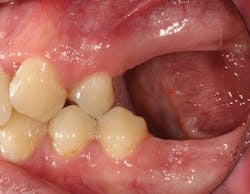
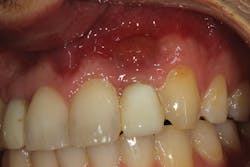
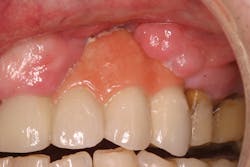
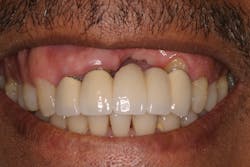
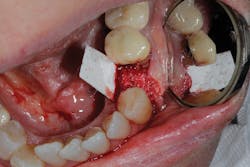
• site development to increase hard and soft tissue for pontic sites in fixed bridge prosthetics (figures 9–14);• correcting bone defects impinging upon anatomical structures after tooth extraction, such as oroantral communication (figure 18); and
• preserving tissue structure for subsequent dental implant therapy.Decision matrix
With these indications in mind, does every extraction socket need to be grafted? The answer is no. A good decision matrix is based on “A Simplified Socket Classification and Repair Technique” by Elian et al.9
Classification when existing tooth is still present
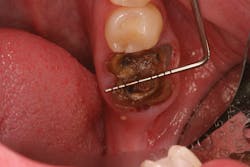
Type 1 socket—Buccal plate present and soft tissue present
• Type 1 socket (figure 19)—Thick biotype, posterior tooth, and buccal plate present: no graft needed
What bone grafting product?
Although there are many types of grafting products commercially available, choosing the right one may be difficult. An ideal bone graft substitute should be biomechanically stable; able to degrade within an appropriate time frame; exhibit osteoconductive, osteogenic, and osteoinductive properties; and provide a favorable environment for invading blood vessels and bone-forming cells.10
Editor’s note: This article originally appeared in Dental Economics’ partner publication Perio-Implant Advisory, a chairside resource for dentists and dental hygienists for issues relating to periodontal and implant medicine. Visit perioimplantadvisory.com to sign up for a newsletter subscription.
Read more about bone grafts in “A review of bone graft material” by Adam Bear, DDS, on the PIA website.
Originally posted in 2019 and updated regularly
References
1. Dye BA, Thornton-Evans G, Li X, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS data brief, No. 197. Hyattsville, MD: National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db197.pdf.
2. Agarwal G, Thomas R, Mehta D. Postextraction maintenance of the alveolar ridge: rationale and review. Compend Cont Educ Dent. 2012;33(5):320-324; quiz 327, 336.
3. Hansson S, Halldin S. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3:1758736012456543. doi:10.1177/1758736012456543.
4. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
5. Lekovic V, Camargo PM, Klokkevold PR, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69(9):1044-1049. doi: 10.1902/jop.1998.69.9.1044.
6. Lam RV. Contour changes of the alveolar processes following extraction. J Prosthet Dent. 1960;10:25-32.
7. Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014;93(10):950-958. doi:10.1177/0022034514541127.
8. Aimetti M, Manavella V, Corano L, Ercoli E, Bignardi C, Romano F. Three‐dimensional analysis of bone remodeling following ridge augmentation of compromised extraction sockets in periodontitis patients: a randomized controlled study. Clin Oral Implants Res. 2018;29(2):202-214. doi:10.1111/clr.13099. Epub November 17, 2017.
9. Elian N, Cho SC, Froum S, Smith RB, Tarnow DP. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;(19)2:99-104; quiz 106.
10. Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury. 2011;42(suppl 2):S77-S81.
11. Mellonig JT, Triplett RG. Guided tissue regeneration and endosseous dental implants. Int J Periodontics Restorative Dent. 1993;13(2):108-119.
12. Araújo Mauricio, Linder E, Wennström J, Lindhe J. The influence of Bio-Oss Collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodontics Restorative Dent. 2008;28(2):123-135.
13. Cardaropoli D, Tamagnone L, Roffredo A, De Maria A, Gaveglio L. Alveolar ridge preservation using tridimensional collagen matrix and deproteinized bovine bone mineral in the esthetic area: a CBCT and histologic human pilot study. Int J Periodontics Restorative Dent. 2018;38(suppl):S29-S35. doi:10.11607/prd.3702.
14. Scheyer ET, Heard R, Janakievski J, et al. A randomized, controlled, multicentre clinical trial of post‐extraction alveolar ridge preservation. J Clin Periodontol. 2016;43(12):1188-1199. doi:10.1111/jcpe.12623.
About the Author

Scott Froum, DDS
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine (SUNY), is a periodontist in private practice in New York City. He is the editorial director of Perio-Implant Advisory and serves on the Dental Economics advisory board. Dr. Froum is a volunteer professor in the postgraduate periodontal program at SUNY, is a trained naturopath, and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him at drscottfroum.com.
Read Dr. Froum's DE Editorial Advisory Board profile here.