Battling for your Business
by Kevin Henry, Managing Editor
For years, Dental Economics® has published articles on the importance of having a good, solid relationship with your dental laboratory. There’s no question that your lab can make your day run smoothly or throw everything off when Mr. Smith’s crown doesn’t fit properly.
Today’s dentist-laboratory relationship can extend across town, the United States, or even the ocean. There have never been more choices in terms of lab choices for dentists ... and the competition for the dentist’s lab dollars has never been greater.
The view from the United States
Industry experts currently estimate that 10 to 20% of U.S. restorative work is fabricated by an offshore laboratory. In a survey conducted by Dental Economics®, nearly 87% of the respondents were not surprised by that number. In fact, one respondent answered, "I’m not surprised by the number. I am surprised that it wasn’t higher."
"Although offshore outsourcing is discussed more openly now than it was just several years ago, the laboratory community is still divided over the issue," said Kelly Fessel Carr, Associate Publisher/Editor, LMT magazine.
"Some laboratory owners embrace the concept because it provides access to additional capacity and the means for growth. Others don’t outsource offshore but accept that it’s part of economic globalization. In a third camp are owners who feel it’s un-American and unfair to employees and are concerned about quality and material controls in foreign countries."
In recent years, "offshore labs" have made a splash, offering lower prices to dentists. Those "lower prices" have been very appealing to doctors with few major problems. However, one of the problems gathered nationwide media attention in 2008 when a 73-year-old Ohio woman being sickened by lead-tainted dental work that was outsourced to China. Though one incident, it’s still a fact that is brought up today in the lab business.
"For us, the FDA concerns about materials coming in from overseas are very real," said Bob Savage, vice president and chief financial officer of Drake Precision Dental Laboratory in Charlotte, N.C. "You have to remember that we’re fabricating body parts. Dentists should be concerned about where their crowns are coming from, and I think they have to know the origin of the product they’re being sold."
Savage not only knows about running a lab in the United States, but is also quick to point out that Drake has an offshore arm operated as Drake Worldwide.
"It’s a separate unit and our customers know up-front what we can do in terms of working at a lower cost with our offshore arm," Savage said.
"We tells our customers the scenario and they know what can be done for what cost. We’re not trying to hide anything, but rather give the customer a choice on pricing."
George Tishakadjian, owner of Divine Dental Studios in North Hollywood, Calif., is adamant on the fact that an inexpensive price tag can result in problems down the road for dentists.
"I don’t do cheap crowns," Tishakadjian said. "I only want to compete with another lab on quality, not price. Cheap labs can’t produce the quality that we can produce. It’s that simple."
He’s also doesn’t hide his feelings that quality includes the materials being used.
"Whatever I’m doing, I know my materials are approved by the FDA," he said.
Savage agrees.
"Ultimately, the dentist is responsible for the work he or she does," Savage said. "Dentists are asked to provide the best possible care for their patients, and that includes having confidence in the materials they use."
So do lab owners believe dentists should be required to tell their patients the origin of their crowns?
"I think that’s an individual decision," Savage said.
"It’s an individual decision that could affect business. If they’re asked by the patient, they should have the confidence to tell that patient exactly where the crown came from. But, on the other hand, if you’re getting an artificial knee, are you going to ask the surgeon where the knee parts came from? That surgeon is a professional just like a dentist. If you don’t trust that person and the decision he or she makes on where to get their materials, why are you going there?"
As part of a survey conducted by Dental Economics®, 78.6% of dentists who responded said that the dentist has an ethical responsibility to tell patients if their work was produced overseas.
"Absolutely every patient should know where his or her crown came from," Tishakadjian said.
And what does the future hold for offshore labs in the mind of the American lab?
"I think we’ll see 20 to 30% of the market fabricated offshore evenutally," Savage said.
"I don’t see it going away or shrinking any time soon. I think the only thing that would slow down its growth would be a large healthcare concern. However, as long as lab owners adhere to guidelines and understand the products that are being brought in, that’s not going to happen."
The view from overseas
Godfrey S.K. Ngai knows the question is coming. Sitting beside the director and chief executive officer of Modern Dental Laboratory (MDL) as we have dinner in Shenzhen, China, I ask him what’s the biggest hurdle his company has to overcome in the mind of American dentists.
"People don’t believe dental labs can be operated in such a fashion as ours," he said after thinking for a moment.
"People try to find every tool they can to attack outsourcing lab cases – poor quality, the lead scare, saying that our people work in sweat shops – it’s simply not true."
Walking the halls of MDL, I saw firsthand that the whispers of second-class conditions are unfounded. Granted, MDL and its 3,000-plus employees may not represent every dental lab in China, but it sets an example of precision and organization.
With a six-building complex in Shenzhen, including four buildings devoted to housing more than 2,000 of its employees, MDL has grown greatly since establishing itself in 1976 with just 45 people. More than 100 people currently comprise the lab’s customer service staff and more than 3,000 units of restorations are produced every day.
"This is a big lab, and that’s what attracted me here at first," said Kenny Tsoi, a technical consultant who has worked 23 years in the lab business, including three at MDL.
"Once I began working here, I learned how important quality is here, in every step of our process. Quality is rechecked at every step, so we know if there is a problem and we can fix it."
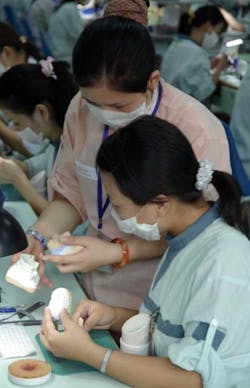
Certified dental technicians working in Modern Dental Laboratory USA’s ISO 13485:2003 certified manufacturing facility in Shenzhen, China.
On this gorgeous October day, Tsoi is leading me and a group of Australian dentists on a tour of the facility. On every floor, technicians are working on cases with amazing focus. There is no music playing. There is no chatting among peers. Technicians concentrate on their work and aren’t distracted by their coworkers or even a mob of visitors walking around them, snapping pictures and chatting.
"What I saw today was much better than I’d expected," said Dr. Michael Stubbs, who practices in Melbourne, Australia.
"It certainly gives a different impression of Chinese dental labs than I had when I walked in here. I was very impressed. After today, I think it’s time to tell everyone that dealing with Chinese dental labs is not a slam on their business."
Modern Dental Laboratory USA chief executive officer Patrick Tessier couldn’t agree more. When he first started working with Modern Dental Laboratory in 2003 (then as Northwest Laboratories), he wasn’t shy about telling his customers that their cases would be sent to China to be processed. It was a hard sell then, but Tessier believes people are quickly warming up to the idea.
"There may be labs that can beat us in cost, but there’s not one who can beat us when it comes to quality," Tessier said. "I’m confident in our people in the United States and China and I’m confident in our product."
American dentists weren’t so confident in overseas dental labs when a lead scare traced to a Chinese dental lab occurred in 2008. Those looking to put a black mark on offshore dental labs still use that incident as a rallying cry, Ngai said.
Modern Dental’s facility in Shenzhen
"We keep invoices for our raw materials for six years; so we know if something goes wrong, we can trace it immediately," Ngai said.
"Check the work done in the United States or anywhere else in the world and you’ll see that there is lead in differing amounts. We believe the product you get from our lab is as safe or safer than anywhere else in the world."
The business model for Modern Dental Laboratory USA is simple. With offices in Chicago and suburban Los Angeles, as well as the headquarters in suburban Seattle, orders come into the U.S. offices and are sent to Shenzhen, where they are translated into Chinese for the lab technicians. The whole round trip to U.S. dentists is about two weeks.
"Our lab works on cases six to eight times longer than other labs because we’re working at a higher and more consistent quality," said Tessier, who says MDL’s ISO 13485:2003 certification should tell customers much about the quality of the organization.
"I believe customers should be working with certified labs. If they’re not, they’re taking a gamble on quality."
Ngai’s business model is simple, and his goal for the immediate future is simple as well.
"We are working hard to make ourselves more visible," he said.
"Once a customer sees our quality, he or she will realize we are not the ones who are light years behind, but actually ahead."
Past DE Issues