Lasers in dentistry: Hard-tissue applications
Scott Froum, DDS
Hector L. Sarmiento, DMD, MSc
Dental laser technology has changed dramatically over the last few decades, transforming the practice of dentistry itself. First described for dental use in 1960,1 light amplification by the stimulated emission of radiation (laser) has increased in use and
application, becoming a much more integral component in the dental field. Popularity in laser use has increased as well because the technological revolution, which has maximized access to information, has raised patient expectations that the medical and dental fields would adopt the newest gadgets.
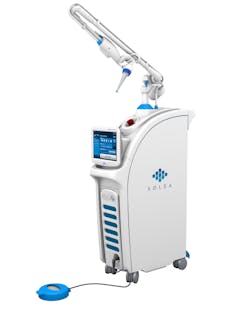
Figure 1: 9.3-micron CO2 laser (Solea, Convergent Dental)
In The Patient Will See You Now: The Future of Medicine Is in Your Hands, author Eric Topol explains that individuals in the millennial and Generation Z populations are going to drive health-care providers to either acquire the latest technology or go out of business.2 Unfortunately, the nebulous topic of lasers in dentistry can be quite confusing, and practitioners claiming to be laser dentists may often misguide the public into thinking that anything and everything can be accomplished with a laser.
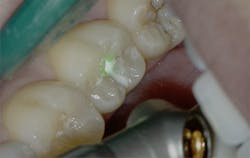
Figure 2: Class I pre-op lesion. Photo courtesy of Jeff Rhode, DDS.
The purpose of this article is to show how lasers can be incorporated into procedures involving hard tissue (enamel and bone) and highlight two commonly used hard-tissue lasers in the dental field.
Lasers used in dental practice can be classified by the lasing medium used, such as gas or solid laser; according to hard- and/or soft-tissue applicability; or according to the range of wavelength. Both the 9,600-nanometer wavelength (9.3-micron) carbon dioxide (CO2) laser (Solea, Convergent Dental) and the 2,940-nanometer wavelength yttrium aluminum garnet (Er:YAG) laser (AdvErL Evo, J. Morita Corp.) are indicated for hard-tissue cutting.
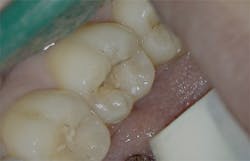
Figure 3: Class I cavity prep after laser. Photo courtesy of Jeff Rhode, DDS.
Both of these hard-tissue lasers are equipped with a handpiece that has a terminal mirror and tips that serve as outlets to deliver the cascade of photons to the hard tissue being targeted. In addition, both lasers have improved delivery of photon energy, because the air-water spray is coaxial to the laser beam. Coaxial existence of air-water enables better delivery of laser energy as well as enhanced cooling and cleansing action. In other words, the hard tissue being cut is cooled by the water spray, which decreases the chance of hard-tissue necrosis due to an increase in tissue temperature. The water spray action clears tissue debris from the field of view, increasing visual perception of the target area.
Figure 4: Class V pre-op lesion. Photo courtesy of Jeff Rhode, DDS.
There are other commonalities between these two lasers. Both can be used to prepare teeth for restorative dental applications, remove stain from teeth, prepare teeth without local anesthesia, remove osseous tissue, and decontaminate bacterial adhesions on dental implants. There are, however, specific differences between the two lasers that may influence a dentist’s decision regarding which laser to purchase.
Figure 5: Class V cavity prep with laser. Photo courtesy of Jeff Rhode, DDS.
9.3-micron CO2 laser
The CO2 laser wavelength has a very high affinity for water, resulting in rapid soft-tissue removal and hemostasis with a very shallow depth of penetration. Most 10.6-micron CO2 lasers are dry lasers with the highest absorbance of any laser in hydroxyapatite, making them excellent soft-tissue lasers but poor hard-tissue lasers, due to overheating and destruction of hard tissue.3 Therefore, the use of these older CO2 lasers is contraindicated for tooth preparation and osseous cutting.
Figure 6: Class V restoration. Photo courtesy of Jeff Rhode, DDS.
For the same reasons, the 10.6-micron CO2 laser has also been shown to damage the implant surface and increase the temperature of the adjacent hard and soft tissue surrounding the dental implant.4 Because of the difference in wavelength, absorption pattern, and introduction of water irrigation with the 9.3-micron CO2 laser (figure 1), it can now be used for hard-tissue applications. This laser has been used to prepare teeth for Class I–IV (figures 2–8) restorations without the concern of raising pulpal temperature and inducing necrosis. The laser has also been used as an alternative to conventional high-speed drills to prepare teeth for crowns, bridges, and veneers.
Figure 7: Class IV lesion. Photo courtesy of Jeff Rhode, DDS.
One of the more appealing features is this laser’s ability to cut teeth without the need for local anesthesia. When this laser is used on the tooth, the cell membranes of the nerve fibers experience a transient loss of conductance of the impulse generated, owing to hyperpolarization culminating in the interruption of the sodium/potassium pump. As a result, pain receptors allied to afferent axons are targeted; hence, a higher intensity is requisite to a normal potential action and an analgesic effect occurs. This analgesia can persist for 15 to 20 minutes and is beneficial for patients who have a phobia of needles as well as young children.
Figure 8: Class IV cavity prep with laser. Photo courtesy of Jeff Rhode, DDS.
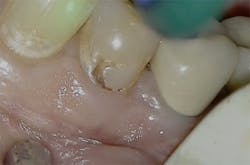
The 9.3-micron CO2 laser can also be used for osteoplasty and ostectomy during periodontal and oral surgeries. The efficient cutting ability and decreased thermal induction of the laser make it a good indication for crown lengthening (figures 9 and 10), osseous contouring, osseous resection, and tooth resection. Ongoing studies have shown this laser to have the ability to decontaminate implants affected by bacterially induced peri-implantitis without damage to the macrosurface of the implant body or an increase in the thermal temperature of the proximal tissue (figure 11).5
Figure 9: Initial presentation prior to crown lengthening
Although there are many advantages to this laser, there are also some disadvantages. First and foremost, the cost of this laser can be prohibitive. Second, this laser has a large footprint and can take up space in a small operatory. Because of the relative recent release of this laser, most of the research showing its surgical ability on hard tissue and dental implants is still in process, and words such as regeneration and equivalency to conventional surgery have not yet been proven. Finally, as with any other laser worth buying, there is a lot of training needed in order to operate this laser at a high level of operator efficiency.
Figure 10: Immediate post-op hard- and soft-tissue crown lengthening with laser
Er:YAG laser
The erbium family of lasers has two distinct wavelengths: Er,Cr:YSGG (yttrium scandium gallium garnet) lasers and Er:YAG (yttrium aluminum garnet) lasers. Er:YAG is a solid active medium where a pure crystal of yttrium aluminum and garnet is intentionally doped with erbium atoms. On prompting, the active medium releases photons in the scope of the wavelength 2,940 nm.6 The erbium wavelengths have a high affinity for hydroxyapatite and the highest absorption of water relative to any dental laser wavelengths.7 This laser is indicated for dental hard tissues and soft-tissue ablation because of its absorption.8
Figure 11: Immediately after laser detoxification of implant by 9.3-micron CO2 laser (1.2 mm spot size low power 20% cutting speed)
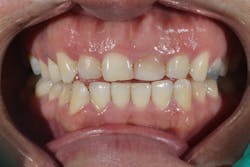
The FDA approved the Er:YAG laser as the first hard- and soft-tissue laser in 1997. The clinical applications for hard tissues first included cavity excavation, enameloplasty prior to root etching, endodontic preparation including root amputation and cutting, shaving, contouring, and resection of oral osseous tissues. It then adopted procedures such as osseous crown lengthening, exostosis removal, and sinus lateral window preparation for sinus augmentation procedures (figures 12–16).
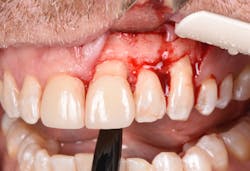
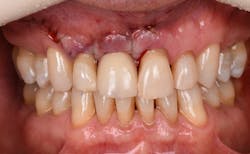
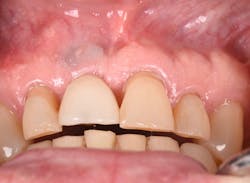
Figure 12: Pre-op clinical photo shows the discrepancies and disharmony of the gingival height. Incisions for esthetic crown lengthening on tooth No. 9 were done via the Er:YAG laser in contact mode (20 pps/60 mJ) to remove excess soft tissue in accordance with the pre-op probing depth measurements.
Figure 13: Exposure of the bony tissues via a full-thickness flap shows osseous irregularities and exostosis ledges that are contributing to the gingival discrepancy. Calculus can be observed attached to the root surface of the teeth.
For soft-tissue procedures, similar to the CO2 laser, its clinical applications include incision, excision, vaporization, ablation, and coagulation of oral soft tissues, including excisional and incisional biopsies. Gingivoplasty, gingival depigmentation, removal of subgingival calculus in periodontal pockets, and sulcular debridement are also treatment modalities for this laser. Its unique attraction to its targeted chromophore allows this laser to ablate both tissues simply by adjusting the pulses per second and millijoules from the unit control panel.
Figure 14: After osseous shaping and recontouring was done using the Er:YAG laser in contact mode (20 pps/80 mJ). All bony ledges and crestal bone were removed on teeth Nos. 9–11.
Figure 15: Immediate post-op of tissues repositioned into place. Simple interrupted sutures were used.
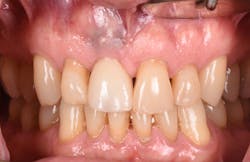
Figure 16: Two-year post-op shows gingival health and maintenance of gingival tissues.
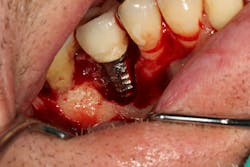
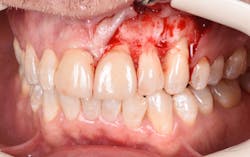
The Er:YAG laser has been more recently known for its ability to remove calculus from a titanium implant surface as well as detoxification (figures 17–21).9 Many authors have shown the laser’s use in combination with mechanical debridement to be superior when using a regenerative approach to treat peri-implant diseases (figures 22–26).10 A particular feature of this laser is the microexplosions that occur when the laser energy is absorbed by water. The volume of these explosions suddenly expand up to 1,000 times, covering more surface area of a titanium implant. It has been found that both contaminated debris and the contaminated oxide layer are removed as a result of these microexplosions.11
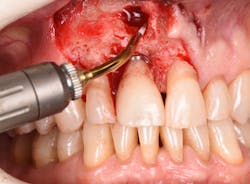
Figure 17: Patient presented with buccal vestibule swelling and complaining of pain. Swelling is visible and palpable with discoloration of the soft tissue.
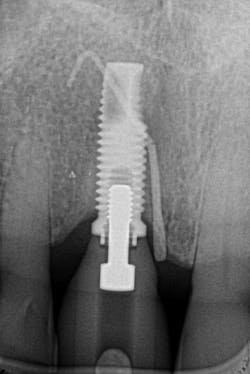
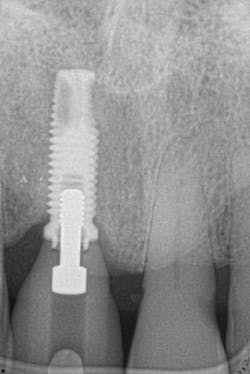
Figure 18: Pre-op radiograph shows retrograde peri-implantitis possibly from previous periapical pathology.
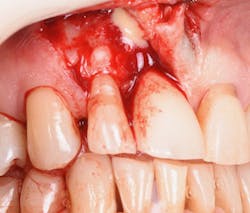
Figure 19: Immediately after incisions and raising a full-thickness flap, suppuration was noted on the apex area of the implant.
A further interesting concept of the Er:YAG laser is the biostimulation phenomenon (photobiomodulation). This biologic effect can promote wound healing and reduce postsurgical inflammation.12 For this reason, the laser can be used for other clinical applications, such as treatment for aphthous ulcer removal, dentinal desensitization, and coagulation properties.
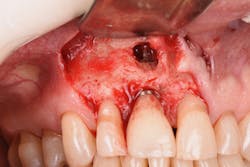
Figure 20: An osteotomy was done to access apical area of implant No. 8 utilizing the Er:YAG laser at 20 pps/70 mJ. The then-exposed area was irradiated using a combination of detoxification and biostimulation of the bone and implant to prepare the area to receive a bone graft.
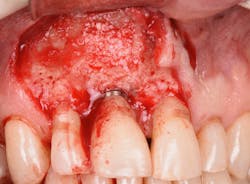
Figure 21: After mechanical debridement and surface detoxification
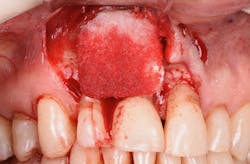
Figure 22: A regenerative approach was taken using a xenograft bone graft in combination with BB human recombinant platelet-derived growth factors.
Purchasing the Er:YAG laser can be beneficial to clinicians who desire a true hard- and soft-tissue laser. The amount of training is minimal, and the settings are similar for various applications. Since the Er:YAG laser has an affinity to water, it has been shown to have minimal thermal changes, and it promotes reduced postoperative discomfort. One study showed that when cutting bone for partially erupted third-molar extractions, not only less intra- and postoperative pain was noted, but also a reduction in facial swelling when compared to conventional bur drilling.13
Figure 23: A porcine 3-D volume stable collagen matrix was used not only as a barrier but also to enhance the thickness of the soft tissue. The matrix thickness measured 3 mm to achieve gingival horizontal volume.
Figure 24: Immediate post-op of gingival tissue repositioned tension-free.
Figures 25 and 26: One-year postsurgery. Clinically all signs of retrograde peri-implantitis have been eliminated and radiographic bone fill can be seen.
References
1. Maiman TH. Stimulated optical radiation in ruby lasers. Nature. 1960;187(4736):493-494.
2. Topol EJ. The Patient Will See You Now: The Future of Medicine Is in Your Hands. New York, NY: Basic Books; 2015.
3. Fujiyama K, Deguchi T, Murakami T, Fujii A, Kushima K, Takano-Yamamoto T. Clinical effect of CO2 laser in reducing pain in orthodontics. Angle Orthod. 2008;78(2):299-303. doi:10.2319/033007-153.1.
4. Geminiani A, Caton JG, Romanos GE. Temperature increase during CO(2) and Er:YAG irradiation on implant surfaces. Implant Dent. 2011;20(5):379-382.
5. Froum SH, Cantor-Balon R, Kerbage C, Froum SJ. Thermal testing of titanium implants and the surrounding ex-vivo tissue irradiated with 9.3 um CO2 laser. In submission.
6. Olivi G, Margolis FS, Genovese MD. Pediatric Laser Dentistry: A User’s Guide. Hanover Park, IL: Quintessence Publications Co Inc.; 2011.
7. Harashima T, Kinoshita J, Kimura Y, et al. Morphological comparative study on ablation of dental hard tissues at cavity preparation by Er:YAG and Er,CR:YSGG lasers. Photomed Laser Surg. 2005;23(1):52-55. doi:10.1089/pho.2005.23.52.
8. Ishikawa I, Aoki A, Takasaki AA. Clinical application of erbium:YAG laser in periodontology. J Int Acad Periodontol. 2008;10(1):22-30.
9. Schwarz F, Rothamel D, Becker J. Influence of an Er:YAG laser on the surface structure of titanium implants. Schweiz Monatsschr Zahnmed. 2003;113(6):660-671.
10. Sarmiento HL, Norton M, Korostoff J, Ko KI, Fiorellini JP. Surgical alternatives for treating peri-implantitis. Int J Periodontics Restorative Dent. 2018;38(5):665-671. doi:10.11607/prd.3639.
11. Yamamoto A, Tanabe T. Treatment of peri-implantitis around TiUnite-surface implants using Er:YAG laser microexplosions. Int J Periodontics Restorative Dent. 2013;33(1):21-30.
12. Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5(1):31-39.
13. Abu-Serriah M, Critchlow H, Whitters CJ, Ayoub A. Removal of partially erupted third molars using an Erbium (Er):YAG laser: a randomised controlled clinical trial. Br J Oral Maxillofac Surg. 2004;42(3):
203-208. doi:10.1016/j.bjoms.2004.02.006.
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice in New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of the American Board of Periodontology, is a clinical associate professor at SUNY Stony Brook School of Dental Medicine in the Department of Periodontology. He serves on the board of editorial consultants for the Academy of Osseointegration’s Academy News. Contact him through his website at drscottfroum.com or (212) 751-8530.
Hector L. Sarmiento, DMD, MSc, is uniquely trained in periodontics and maxillofacial surgery. He is board-certified in periodontics. He is a clinical associate professor at the University of Pennsylvania in the Department of Periodontics. Dr. Sarmiento lectures internationally and focuses his research on bone biology and the treatment of peri-implantitis. His private practice is located on the Upper East Side of Manhattan, New York.