The Perio/Systemic Link
The consensus in the medical and dental professions is that there is an association between the mouth and the body. Even without bulletproof evidence of the perio/systemic links at this time, it is apparent that we are not only treating mouths. If we educate our patients about the critical importance of meticulous plaque control and professional care, we can significantly impact their general health.
This is a uniquely fascinating time to be a dental professional. Research around the world into virtually all aspects of the profession is occurring at an unprecedented rate. Among the most significant findings of this research is the emergence of the link between periodontal disease and a variety of conditions in the body.
As dental professionals, we have a clinical, ethical, and legal responsibility to inform our patients - and by extension, the general public - about the impact of oral health on systemic health. As such, it is important for us to understand the sequence of events beginning with periodontal pathogens, development of periodontal disease, and the relationship to elevated risk of systemic events. Dental professionals have a high level of credibility because of our education. Hand in hand with that credibility we enjoy is an enormous responsibility to provide appropriate treatment to every patient, every time they are in our offices - not just for their oral health, but for their general health as well.
It is now universally accepted that periodontal disease is a bacterial infection caused by a specific group of bacterial species aggregated on biofilms. Dental plaque is a biofilm; in fact, it is the most widely studied biofilm in the world. Biofilms are found everywhere including rivers, streams, plumbing lines, ship hulls, and nuclear power plants. It is the slimy stuff in a vase after flowers have been in it for several days. Many different organisms can form biofilms, which are responsible for a wide variety of diseases. By some estimates, 65 percent of all diseases are biofilm-induced, including diseases such as pneumonia, tuberculosis, Legionnaires disease, periodontal disease, and subacute bacterial endocarditis. SBE is a biofilm-induced, perio/systemic link. Oral bacteria enter into the circulatory system and have the potential to lodge on the heart valves. This is a direct perio/systemic link.
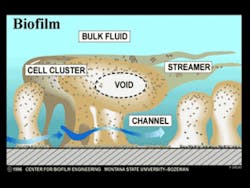
Biofilm develops in stacked layers of different bacterial species. It does not grow by one species of bacteria colonizing the pellicle and then growing large numbers of that one bacterial species. The early biofilm colonizers are predominantly Actinomyces and Streptococcus species. These early colonizers are not pathogenic; i.e., they cannot cause periodontal disease. They can cause gingivitis. As the early colonizing bacteria are coming onboard the biofilm, they release small amounts of signaling molecules, primarily in the form of peptides. These signaling molecules indicate to other bacteria that are freely floating around in the mouth that the conditions on the biofilm are now favorable for them to come onboard. The next group of bacteria also releases signaling molecules, and the biofilm continues to grow in a sequential fashion. The later biofilm colonizers are the periodontal pathogens. Currently, four species of bacteria are consistently associated with periodontal disease: Porphyromonas gingivalis, Treponema denticola, Tannerella forsythensis, and Actinobacillus actinomycetemcomitans. Other bacteria have been variably identified as perio pathogens as well (Figure 1).
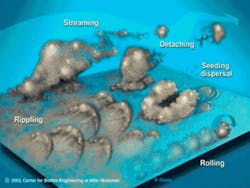
As the biofilm grows, fluid channels develop. These fluid channels or streams direct the one liter of saliva secreted daily, or any beverages ingested, around the biofilm, bringing nutrients to the bacteria and removing waste products. Voids in the interior of the biofilm direct these fluids to the interior so that bacteria do not have to be only on the surface to have nutrient delivery and waste removal. These fluid forces have other very significant effects as well. The fluid forces influence the architecture of the biofilm and the spatial arrangement of the bacteria within. They also enhance certain metabolic processes such as genetic transfer among bacteria. Plaque is not a dome-shaped structure over which the fluids passively flow. The bacterial colonies in biofilm are forced into a variety of shapes and events by the fluids, such as rippling and rolling (Figure 2). These fluid forces also detach portions of the biofilm, some of which are swallowed or expectorated. Other detached portions, however, form new biofilm colonies in another area of the mouth. Every time bacteria collide with each other, a transfer of genes occurs. The fluid forces increase the rate of bacterial cell collisions. This results in an increased rate of genetic transfer among the bacteria. The increased genetic exchange means that the biofilm bacteria are constantly evolving. This continuous evolution is a primary defense mechanism, ensuring their survivability in the oral cavity, which is a very hostile environment. This is one of the mechanisms responsible for the extremely tenacious property of the oral flora.
Oral bacteria undergo different metabolic events depending on their location in the mouth. Bacteria aggregated on biofilm produce hundreds of proteins that free-floating bacteria do not. Some of these proteins are signaling molecules that trigger genetic activity, which results in a variety of events such as bacterial aggregation, matrix formation, and adhesion to other biofilm bacteria. The same species of bacteria do not produce these signaling molecules when they are freely floating in the mouth. This means that the geography determines the metabolism, such that the location of bacteria in the oral cavity significantly determines the metabolic processes they undergo.
Recent research has revealed that in order to cause periodontal disease, bacteria must be attached to something. Free-floating bacteria, regardless of its pathogenicity cannot cause periodontal disease. In the gingival crevice, bacteria will adhere to the tooth surface, the epithelial lining of the pocket, or other bacteria already on the tooth or epithelium. There are binding sites on all of these to which the bacteria can adhere.
We have known for a long time that periodontal disease develops where plaque accumulates. We now know, however, that the reason periodontal disease develops where plaque accumulates is not because the amount or volume of plaque is greater in these locations. We can have a large population of the early colonizing species, and yet only have gingivitis develop because the early colonizers cannot cause periodontitis. The reason disease develops where plaque accumulates is because the biofilm has not been removed, or at least adequately disrupted. The perio pathogens have had a chance to come out of their free-floating locations and colonize the biofilm, becoming the predominant species. The reason periodontal disease develops where plaque accumulates is because the species of plaque bacteria has shifted from the early colonizing bacteria to the perio pathogens. This is called the ecological plaque hypothesis.
We can never attain a germ-free mouth, because there are billions of microorganisms in the mouth. But we can attain a germ-balanced mouth. In a healthy mouth devoid of periodontal disease, the early colonizers and the perio pathogens are in balance. In fact, the early colonizers are considered favorable, or beneficial. They can cause a low-level gingival inflammation, which results in a low-level immune system response. This immune response helps to keep the perio pathogens in check. The immune system response to the gingivitis is the reason early colonizers are considered favorable. If the perio pathogens overwhelm the early colonizers and the immune response, periodontal disease will manifest. A germ-balanced mouth exists when the patient has periodontal health.
Oral bacteria in the gingival crevice are in direct physical contact with the pocket epithelium, which presents an opening into the circulation. When bacteria are in the gingival crevice, they are not just occupying the space between the tooth and the epithelium. They can rapidly enter the gingival epithelial cells and migrate through the epithelium into the underlying connective tissue. This intimate contact of the bacteria with the epithelium results in bacteria entering the circulation through blood vessels in the gingiva. The resulting bacteremia consists of live bacteria and their endotoxins. When bacteria break down, their cell walls break down into lipids, carbohydrates, and proteins. These components are the endotoxins.
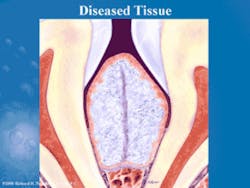
The body responds to the bacteremia by mounting an inflammatory response. A variety of inflammatory mediators and enzymes are released to combat the bacteria and endotoxins. The enzymes released by the body include collagenase, gelatinase, elastase, and proteases. The enzyme activity is implied by their names, so that collagenase destroys collagen, which is the main structural component of the periodontium. Proteases destroy proteins, and gelatinase destroys the gelatinous matrix that surrounds the biofilm. Previous theories of gingival tissue destruction indicated that the bacteria were releasing acids that severed the fibrous gingival attachment apparatus. We now know that the majority of periodontal tissue destruction occurs because the body is doing it to itself, through the inflammatory response (Figures 3 and 4). The bacteria release a variety of toxins, metabolites, and enzymes that cause some tissue degradation, but current research indicates this is the minor cause of perio tissue destruction.
Next in the sequence of immune system events, the liver releases a protein called C-reactive protein (CRP). CRP is released by the liver in response to injury, inflammation, or infection. It is an attractor of the inflammatory mediators. CRP goes from the liver to the site of the injury, inflammation, or infection and attracts the inflammatory mediators which then combat the injurious agent. Recent research indicates that C-reactive protein is an active participant in atherogenesis; i.e., the development of clots and plaques in blood vessels. The development of atheromatous plaques and clots constitutes a mechanism of increased risk of cardiovascular events such as heart attack and stroke.
The inflammatory response to periodontal disease has been implicated by a large body of research in other systemic events. Women with periodontal disease appear to be four to eight times more likely to have a premature, low-birth-weight baby. Oral microbes have been shown to cross the placenta, exposing the fetus to infection. Several mechanisms of the increased rate of premature infant delivery have been advanced. One mechanism is the ability of the inflammatory mediators to cross the placenta, causing fetal toxicity resulting in a preterm, low-birth-weight infant. Another mechanism identified is due to fetal exposure to oral bacteria, evidenced by a fetal immune response. This exposure has been associated with the delivery of a preterm infant. Other studies demonstrate that periodontal treatment may reduce the incidence of adverse pregnancy events.
In the dental profession, we have known for more than 50 years that diabetic individuals are predisposed to periodontal disease. We now know that diabetes and periodontal disease affect each other. Research has shown that the presence of periodontal disease makes it more difficult to maintain adequate glycemic control. Some studies have indicated, however, that when periodontal disease is successfully treated, glycemic control is improved in type 2 diabetics. Other studies have demonstrated significant improvements in HbA1c levels three and six months after periodontal disease is treated.
The consensus in the medical and dental professions at this time is that there is an association between the mouth and the body. The level of causality is still up for debate among some researchers and clinicians. Further research, particularly interventional studies, will likely demonstrate that periodontal disease is one of several factors involved in a variety of systemic diseases and conditions.
Even without bulletproof evidence of the perio/systemic links at this time, it is apparent that we are not treating only mouths. If we educate our patients about the critical importance of meticulous plaque control and professional care, we can significantly impact their general health. If we treat every patient, every time they present to our care, we can potentially improve blood sugar control for our diabetic patients. We can possibly help reduce the incidence of premature, low-birth-weight infants for our patients who are pregnant. It is our responsibility to educate our patients who have other cardiovascular risk factors - such as smoking, sedentary lifestyles, poor diets, and a family history of heart attack and stroke - about the increased risk of cardiovascular events from their periodontal disease.
This is truly an exciting time to be in the dental profession. As clinicians, we are in a unique position to positively impact the total health of everyone under our care. New treatment modalities will become available to us in the not-too-distant future. The convergence of medicine and dentistry is upon us. We must pay close attention to the rapid pace of research that enables us to deliver the most comprehensive care for our patients. And that is what it is all about - the care we provide for every patient, every time.Richard H. Nagelberg, DDS, PC, is in full-time practice in Plymouth Meeting, Pa. He is a 1982 graduate of Georgetown University, School of Dentistry. Visit Dr. Nagelberg’s Web site at www.richardnagelberg.com, or e-mail him at [email protected].